History
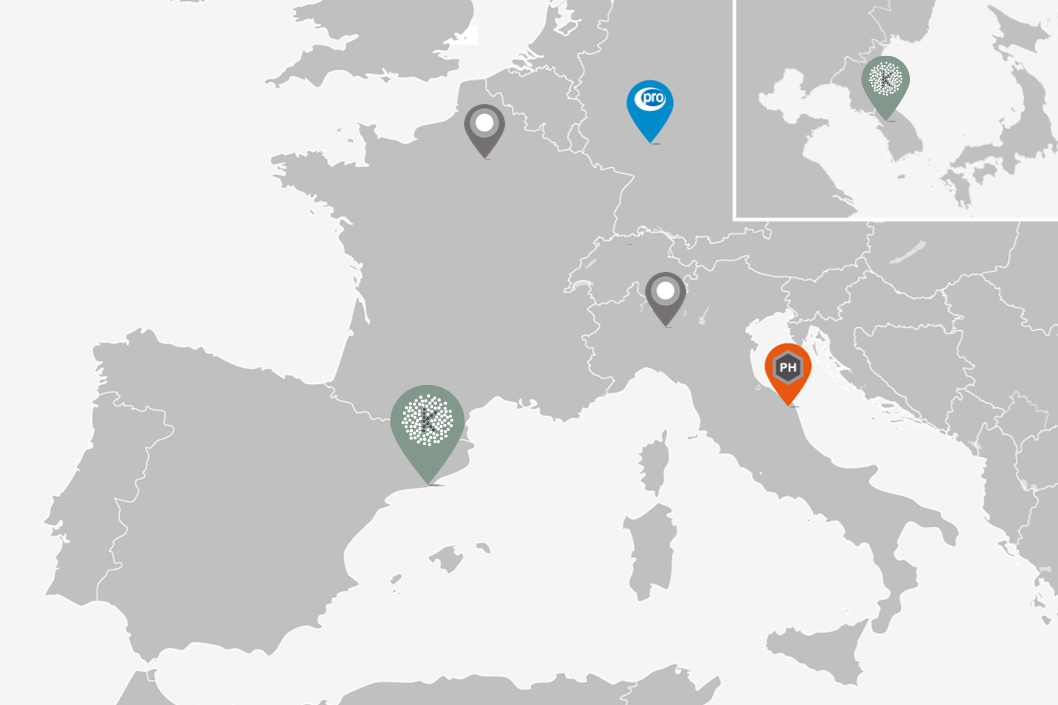
Kymos S.L. was founded in 2001 by a team of R&D scientists with decades of experience in pharmaceutical development from early research to final formulation and scale up trials for commercialization. The company was established at the Barcelona Science Park and rapidly expanded despite high competition. In 2011 Kymos took over Ipsen Pharma’s immunology team to create a pioneering biological division. In 2015 Kymos moved to a brand-new laboratory at the Vallès Technology Park. In 2016 Kymos acquired Italian CRO Pharmaprogress. In 2018, steady growth called for two successive extensions with a new office building and laboratories. In 2020 Kymos acquired German CRO Proytic. In 2021 the Kymos Group is made up of a two-building HQ plus two European sites, 14 laboratory departments, 170 highly trained experts and 5,000 m2 worth of facilities and top-of-the-line instruments.